ACCME Accreditation Statement
Coming soon – July 2026 | ASPN | Location: TBD
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the Congress of Neurological Surgeons (CNS) and the North American Neuromodulation Society (NANS). The Congress of Neurological Surgeons is accredited by the ACCME to provide continuing medical education for physicians.
AMA Credit Designation Statement(s)
The CNS designates this live activity for a maximum of 20.75 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
CREDIT FOR NURSES AND NURSE PRACTITIONERS
American Nurses Credentialing Center
The American Board of Neuroscience Nursing (ABNN)
The American Board of Neuroscience Nursing (ABNN) accepts attendance at educational activities approved for CME credit for CNRN or SCRN recertification. The educational activity must be specific to neuroscience or stroke, and a course agenda may be required for any sessions that do not explicitly indicate neuroscience content. Any item that is not neuroscience or stroke-related will not be accepted.
As needed, you can use the formula below to convert continuing education credit or academic hours:
1 AMA PRA Category 1 Credit™ credit hour = 1 CE Hour
Check the following web pages for additional requirements: CNRN and SCRN. For more information regarding recertification requirements, please contact the ABNN directly at Phone: 847-375-4733 or Email: info@abnncertification.org
Acceptable supporting documentation
All education submitted in the recertification application at www.abnncertification.org, must include the following information:
Title of the program
Provider – the organization that is administering the program
Number of credits
Sponsor – the organization that designates the program for CME credit
If the course title(s) does not reflect the content, you must provide a brief description of the content. Keep documentation of all completed activities for 7 years in case you are audited by the ABNN.
CREDIT FOR PHYSICIAN ASSISTANTS
The American Academy of Physicians Assistants (AAPA)
The National Commission on Certification of Physician Assistant (NCCPA)
The National Commission on Certification of Physician Assistant (NCCPA) accepts Category 1 CME credit for activities designated by the AMA PRA Category 1 Credit ™ from organizations accredited by the Accreditation Council on Continuing Medical Education (ACCME).
Through joint Providership with the CNS, the 2025 North American Neuromodulation Society Annual Meeting, meets the criteria for acceptable AAPA Category 1 CME.
As needed, you can use the formula below to convert continuing education credit or academic hours:
1 AMA PRA Category 1 Credit™ credit hour = 1 Category 1 CME Credit
Acceptable supporting documentation
Acceptable supporting documentation for Category 1 credits includes the certificate. The documentation should indicate the PA’s name, the accredited ACCME organization’s name, the CME activity’s title and date, and the number of credits designated for Category 1 Credit.
NCCPA provides the option to upload CME certificates when logging in, but you are not required to submit documentation at the time that you log credits. However, be sure to keep documentation of all completed Category 1 CME activities for 7 years in case you are audited by NCCPA.
Check with your state licensing board regarding their individual auditing and documentation requirements.
For more information regarding recertification requirements, please contact the NCCPA directly: National Commission on Certification of Physician Assistants (NCCPA) Phone: 678.417.8100 Email: nccpa@nccpa.net
Evaluation and Credit Claim
The CNS designates this live activity for a maximum of 20.75 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Meeting participants may also claim attendance hours for the meeting.
An overall meeting evaluation will be required to claim credit and attendance hours. Instructions on how to access the Credit Claim site will be provided on the first day of NANS 2025 on the meeting website and meeting app. The deadline to claim credits and attendance hours is May 1, 2025.
If you have any questions, please contact NANS at education@neurodulation.org.
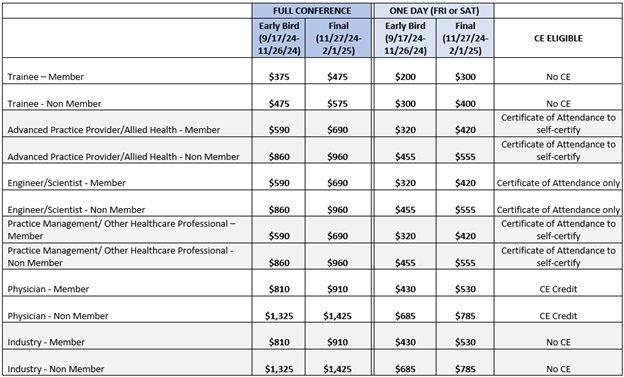
2025 Registration Fees & CE Eligibility
Disclosure Policy
In accordance with the ACCME Accreditation Criteria, the Congress of Neurological Surgeons, as the accredited provider of this activity, must ensure that anyone in a position to control the content of the educational activity has disclosed all financial relationships with any ineligible company. Therefore, it is mandatory that both the program planning committee and speakers’ complete disclosure forms. Members of the program committee and speakers were required to disclose all financial relationships. The ACCME defines an ‘ineligible company’ as “companies whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.” The ACCME considers financial relationships as financial transactions (in any amount) that may create a conflict of interest and occur within the 24 months preceding the time that the individual is being asked to assume a role controlling content of the educational activity. The disclosure to learners will be available on the meeting website and the meeting app.
FDA Statement
Some drugs or medical devices demonstrated at the NANS 2025 Annual Meeting have not been cleared by the FDA or have been cleared by the FDA for specific purposes only. The FDA has stated that it is the responsibility of the physician to determine the FDA clearance status of each drug or medical device he or she wishes to use in clinical practice. Through the joint providership of CNS and NANS, NANS follows CNS policy. CNS policy provides that “off label” uses of a drug or medical device may be described at the Annual Meeting so long as the “off label” use of the drug or medical device is also specifically disclosed. Any drug or medical device is “off label” if the described use is not set forth on the product’s approval label. It is also each speaker’s responsibility to include the FDA clearance status of any device or drug requiring FDA approval discussed or described in their presentation or to describe the lack of FDA clearance for any “off label” uses discussed. Speakers from the audience are also required, therefore, to indicate any relevant personal/professional relationships as they discuss a given topic.